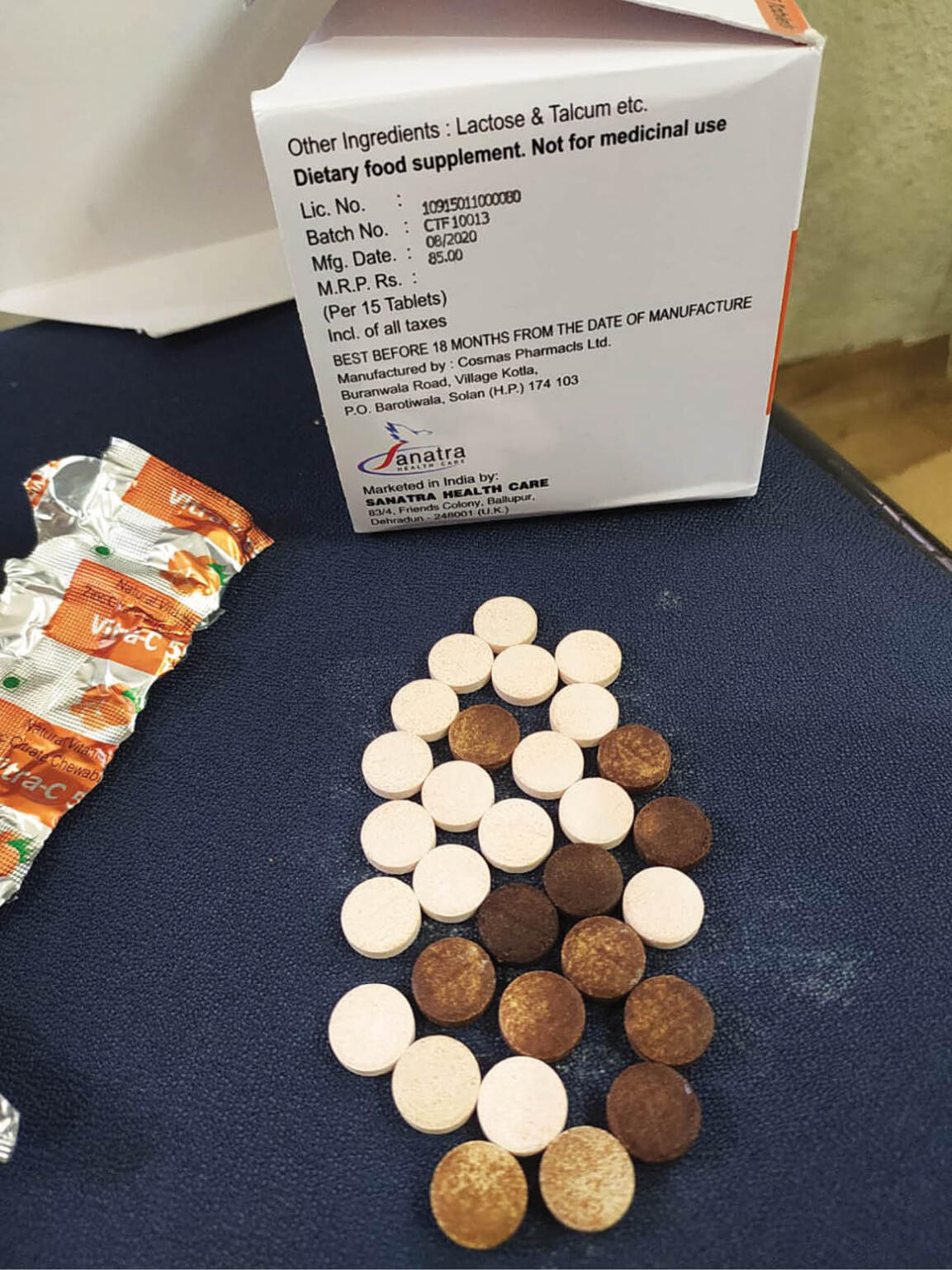
Vitamin-C ṭha tâwk lo man
Health Services department hnuaia Food & Drug Administration (FDA) chuan nimin khan Kolasib-ah vitamin-C ṭha tâwk lo Vitra C 500 eng emaw zat an man a, a ṭul angin hma an la nghal.
FDA joint director Lalsawma chuan Vanglaini palai zawhna chhangin, Vitra C 500 ṭha tâwk lo Mizoramah a darh hman tawh viau thei tih a sawi a, "Uttarakhand-a manufacturing company-te siam a ni a, a formulation a dik lo nasa. A rawng a inthlâk nasa a, mihring tan ei ngam chi a ni lo," a ti.
Lalsawma chuan hma an lâk nghal thu sawiin, "A siamtu company-te hian an pek chhuah tawh zawng zawng an la lêt dawn a, chumi tur chuan hma an la ang," a ti a; company-in hma an lâk ṭhat duh loh chuan an thil siam chhuah dangte pawh Mizorama zawrh khap tura hmalak a nih tur thu a sawi.
FDA chuan, damdawi zuartute chu Vitra-C 500 tablet an neihte endik a, ṭha tâwk lo an kawl a nih chuan an laknaah kîr vek turin an hriattir a ni.